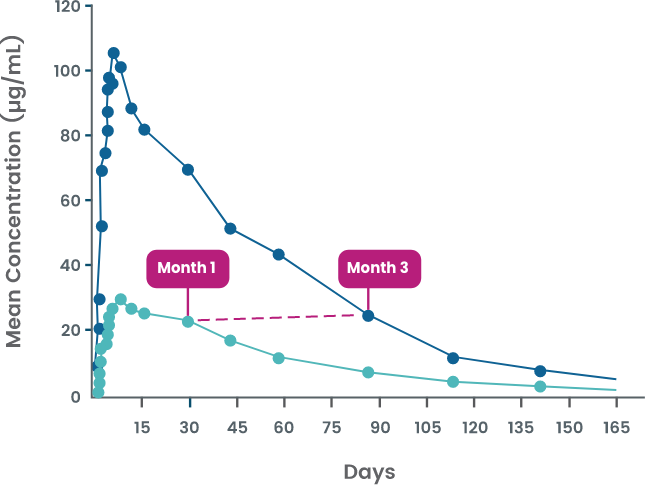
AJOVY was evaluated in a phase 1 study to assess pharmacokinetic parameters for both monthly and quarterly dosing in healthy volunteers.4
Serum concentrations of AJOVY were sustained throughout both the monthly and quarterly dosing periods.5
- Once steady state is reached, the washout period† for AJOVY is similar for monthly and quarterly dosing (~5-6 half-lives)6
†Washout period defined as time until plasma concentrations of AJOVY are below a clinically relevant concentration and thus considered eliminated.