Contraindications: AJOVY® is contraindicated in patients with serious hypersensitivity to fremanezumab-vfrm or to any of the excipients. Reactions have included anaphylaxis and angioedema.
Hypersensitivity Reactions: Hypersensitivity reactions, including rash, pruritus, drug hypersensitivity, and urticaria were reported with AJOVY in clinical trials. Most reactions were mild to moderate, but some led to discontinuation or required corticosteroid treatment. Most reactions were reported from within hours to one month after administration. Cases of anaphylaxis and angioedema have been reported in the postmarketing setting. If a hypersensitivity reaction occurs, consider discontinuing AJOVY and institute appropriate therapy.
Adverse Reactions: The most common adverse reactions in clinical trials (≥5% and greater than placebo) were injection site reactions.
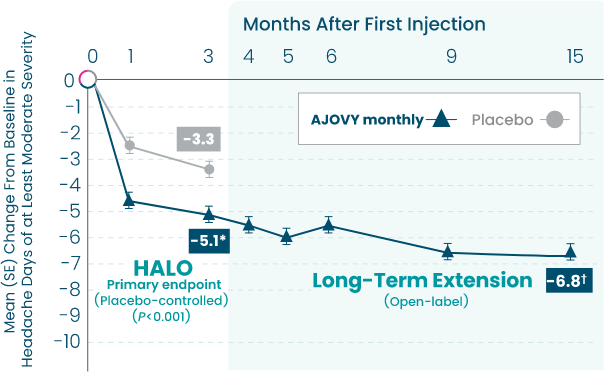
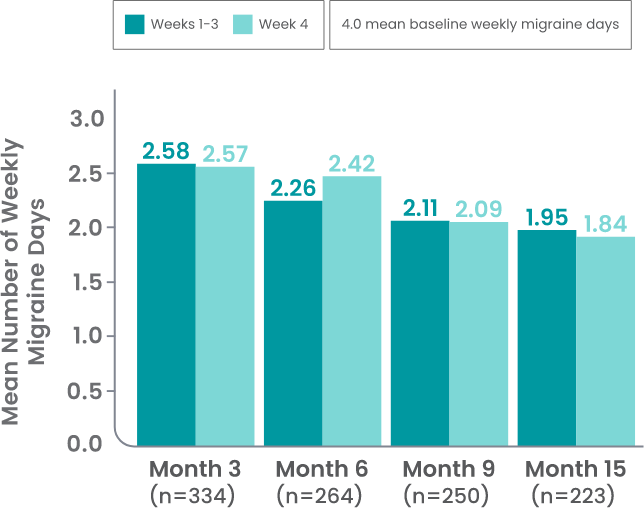
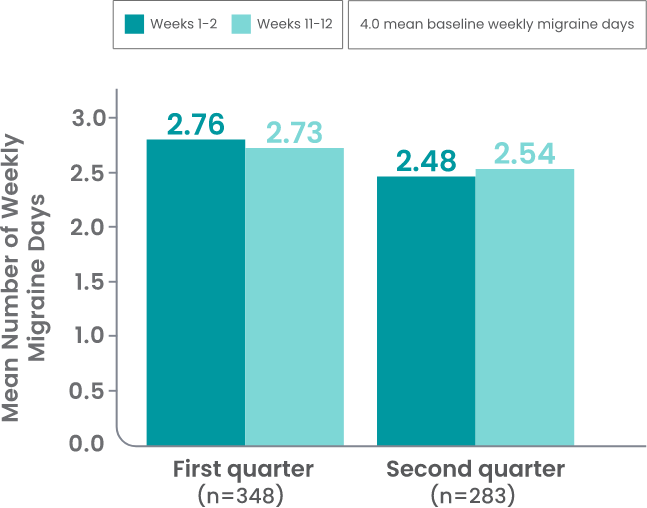
AJOVY is indicated for the preventive treatment of migraine in adults.
Please see full Prescribing Information for AJOVY.